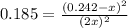Advanced Placement (AP), 26.02.2021 16:40 jdvazquez18p7a7vs
For the equilibrium: 2 NO (g) ⇌ N 2(g)+ O 2(g)at 300 K, the equilibrium constant, Kc, is 0.185. If 1.45 moles each of N 2(g)and O 2(g)are introduced in a container that has a volume of 6.00 liters and allowed to reach equilibrium at 300 K, what are the concentrations of N 2(g ) , O 2(g) ,and NO (g)at equilibrium?

Answers: 1
Another question on Advanced Placement (AP)

Advanced Placement (AP), 23.06.2019 22:30
Need with my drivers ! ** willl mark you brainliest if answered correctly ** when the center line is one solid yellow line and one broken yellow line, who may cross the line to pass? a. traffic on the side with the broken yellow line b. traffic on the side with the solid yellow line c. traffic on either side d. nobody
Answers: 1

Advanced Placement (AP), 25.06.2019 17:30
Identify one similarity between china and europe that explains their decisions on wether to explore or conquer lands around the indian ocean
Answers: 1

Advanced Placement (AP), 26.06.2019 04:00
Analyze the misfit’s statement: “she would have been a good it had been somebody there to shoot her everyday of her life.”
Answers: 1

Advanced Placement (AP), 26.06.2019 08:30
Which side did jupiter chose in the trojan war
Answers: 1
You know the right answer?
For the equilibrium: 2 NO (g) ⇌ N 2(g)+ O 2(g)at 300 K, the equilibrium constant, Kc, is 0.18...
Questions

History, 30.07.2019 20:10

French, 30.07.2019 20:10

Biology, 30.07.2019 20:10


Mathematics, 30.07.2019 20:10


Mathematics, 30.07.2019 20:10


English, 30.07.2019 20:10











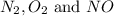 at equilibrium are 0.112 M, 0.112 M and 0.260 M
at equilibrium are 0.112 M, 0.112 M and 0.260 M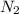 = 1.45 mole
= 1.45 mole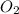 = 1.45 mole
= 1.45 mole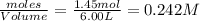
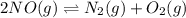
![K_c=\frac{[N_2]\times [O_2]}{[NO]^2}](/tpl/images/1150/8878/68b6f.png)