
Advanced Placement (AP), 08.04.2021 21:50 manlycool7543
Question 1:
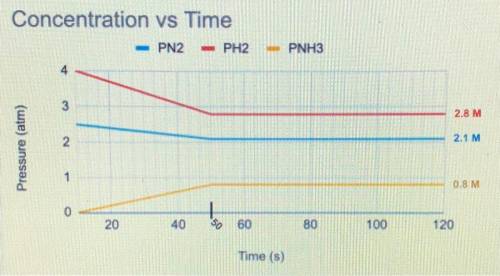
The balanced equation below is a reversible reaction. The concentration of each chemical species was
measured at various time points.
N2(g)+3 H2 (g) <—> 2NH3 (g)
a) When was equilibrium reached?
b) Write the equilibrium expression.
c) Would you expect the Keg to be greater than or less than 1? Justify your answer using data from the graph.

Answers: 2
Another question on Advanced Placement (AP)

Advanced Placement (AP), 23.06.2019 14:00
How can firms use marginal analysis to determine the price of a product? a) profit maximization can occur when the marginal cost exceeds the marginal revenue. b) the total cost of production can determine the individual unit price of a product. c) marginal analysis can be used to determine at what price profit maximization occurs. d) firms can calculate the marginal cost of a product and set the price lower than the cost.
Answers: 2

Advanced Placement (AP), 25.06.2019 21:30
If you have a green turn signal light, you must before making your turn. a. check the crosswalk b. yield to cross traffic and check the crosswalk c. turn off your car's turn signal d. none of the above
Answers: 2

Advanced Placement (AP), 25.06.2019 23:00
Which of the following is a reason to approach smaller banks for a business loan? question options: their criteria for approving a loan are much less stringent than those for larger banks. they emphasize personal relationships and may give more weight to personal attributes. they have lower fees. they grant larger loans.
Answers: 2

Advanced Placement (AP), 26.06.2019 00:00
Which best describes the t in a smart goal?
Answers: 1
You know the right answer?
Question 1:
The balanced equation below is a reversible reaction. The concentration of each chemica...
Questions


History, 18.10.2019 03:30


English, 18.10.2019 03:30

History, 18.10.2019 03:30

Physics, 18.10.2019 03:30

Mathematics, 18.10.2019 03:30



Mathematics, 18.10.2019 03:30

Mathematics, 18.10.2019 03:30





Mathematics, 18.10.2019 03:30

Business, 18.10.2019 03:30

Mathematics, 18.10.2019 03:30


History, 18.10.2019 03:30



