
Advanced Placement (AP), 27.12.2019 19:31 hails271
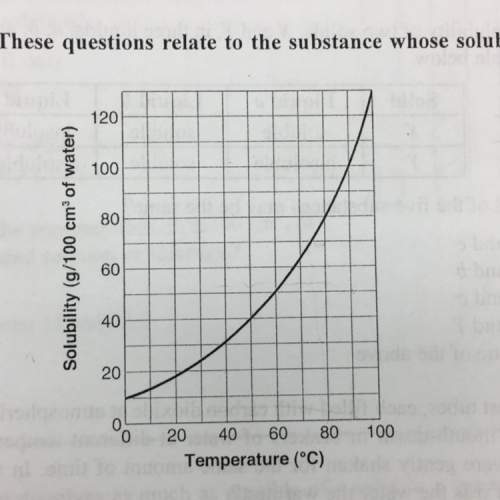
Suppose that 70g of a substance is dissolved in 100cm^3 of water at 80°c. how much of the substance, in grams, will precipitate when the solution is cooled down to 30°c?
a. 55
b. 45
c. 10
d. 30
e. 25

Answers: 2
Another question on Advanced Placement (AP)

Advanced Placement (AP), 22.06.2019 06:30
Imagine a correspondence between thomas hobbes and john locke. first, write a letter from hobbes to locke in which hobbes argues that absolute monarchy is the ideal form of government. then write a second letter from the perspective of john locke in which he answers and refutes hobbes’s argument. even though both hobbes and locke lived during the 17th century and would not have been aware of the enlightened despots the 18th century, be sure to mention how hobbes and locke might have felt about the possibility of these rulers.
Answers: 3

Advanced Placement (AP), 23.06.2019 07:20
Which of the following is not a feature of the surface mining control and reclamation act? regulation of surface coal mines repayment of loss of benefits to ill mine workers restoration of lands to pre-mining conditions retroactivity return of land to original topography
Answers: 3

Advanced Placement (AP), 23.06.2019 08:30
The 529 plan is the type of plan. a. financial fid. b. grant. c. tuition savings. d. scholarship
Answers: 1

Advanced Placement (AP), 24.06.2019 22:30
Gneiss is formed. the mineral grains in which granite are flattened under pressure
Answers: 1
You know the right answer?
Suppose that 70g of a substance is dissolved in 100cm^3 of water at 80°c. how much of the substance,...
Questions

Biology, 19.01.2021 14:00


Mathematics, 19.01.2021 14:00


Mathematics, 19.01.2021 14:00

History, 19.01.2021 14:00

Mathematics, 19.01.2021 14:00




Mathematics, 19.01.2021 14:00

Biology, 19.01.2021 14:00

History, 19.01.2021 14:00


Mathematics, 19.01.2021 14:00

History, 19.01.2021 14:00

Computers and Technology, 19.01.2021 14:00

History, 19.01.2021 14:00

History, 19.01.2021 14:00




