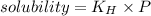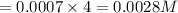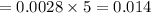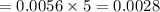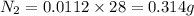The concentration of n2 in blood at 37 °c (body temperature) and atmospheric pressure (partial pressure of n2 = 0.80 atm) is 0.00056 mol/l. a deep-sea diver breathes compressed air with the partial pressure of n2 equal to 4.0 atm. assume that the total volume of blood in the body is 5.0 l. calculate the mass and volume (at 37°c and atmospheric pressure (p(n2) = 0.80 atm) of n2 gas released as a diver surfaces.

Answers: 2
Another question on Biology

Biology, 21.06.2019 15:00
What is a coast of using technology to transport water? a.cities can be built in otherwise uninhabitable areas. b.crops can be irrigated and grown in new areas. c.floods can be averted in flood-prone areas. d.freshwater supplies can be depleted in an area.
Answers: 2

Biology, 21.06.2019 23:30
The organelle pictured is found in cells of and is theorized to have once been an independent organism.
Answers: 3


Biology, 22.06.2019 04:00
Select the true statements about eubacteria. most live as decomposers and heterotrophs. most only thrive in a narrow range of environments. certain eubacteria are responsible for food poisoning. eubacteria thrive in extreme environments.
Answers: 3
You know the right answer?
The concentration of n2 in blood at 37 °c (body temperature) and atmospheric pressure (partial press...
Questions



Mathematics, 31.01.2020 03:52

History, 31.01.2020 03:52


History, 31.01.2020 03:52


Mathematics, 31.01.2020 03:52




English, 31.01.2020 03:52

Mathematics, 31.01.2020 03:52

Mathematics, 31.01.2020 03:52


History, 31.01.2020 03:52

Mathematics, 31.01.2020 03:52

Chemistry, 31.01.2020 03:52