What is shown in the image?
answer options:
prokaryote
eukaryote
ch...

Biology, 13.01.2020 19:31 jamtil7937
What is shown in the image?
answer options:
prokaryote
eukaryote
chloroplast
mitochondrion
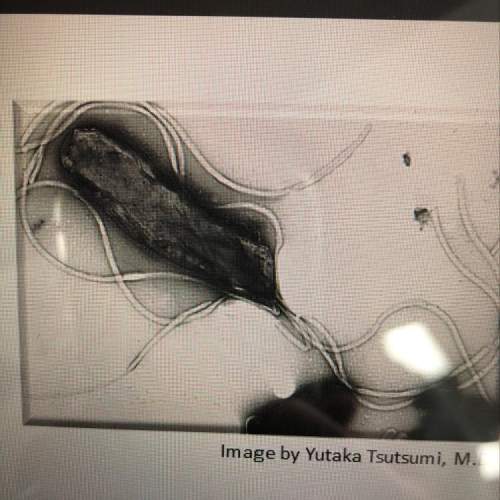

Answers: 3
Another question on Biology

Biology, 22.06.2019 04:30
What is the similarities and differences between bacteria and eukaryote?
Answers: 3

Biology, 22.06.2019 07:00
Common symptoms of an iron-defiency anemia include muscle weakness shortness of breath and lightheadedness why does iron deficiency causes these symptoms
Answers: 2

Biology, 22.06.2019 08:40
What best explains whether bromine (br) or neon (ne) is more likely to form a covalent bond? bromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfills the octet rule. bromine forms covalent bonds because it has many electron shells, but neon has only two electron shells and is tightly bound to its electrons. neon forms covalent bonds because it can share its valence electrons, but bromine has seven valence electrons and can gain only one more electron. neon forms covalent bonds because it has only two electron shells, but bromine has many electron shells and will lose electrons in order to fulfill the octet rule.
Answers: 3

Biology, 22.06.2019 09:00
What is the significance of the protein-lined pits? a. protein attracts other proteins needed for atp synthesis within the cell. b. protein-lined pits are able to transport one molecule at a time down the concentration gradient within the cell. c. the polarity of proteins allows other polar molecules to attach and be transported in the cell by transport channels. d. receptors within the pits allow ligands to fuse and be transported into the cell by endocytosis.
Answers: 2
You know the right answer?
Questions



Mathematics, 09.04.2021 01:50







Mathematics, 09.04.2021 01:50

Mathematics, 09.04.2021 01:50

Mathematics, 09.04.2021 01:50


Social Studies, 09.04.2021 01:50

Health, 09.04.2021 01:50




History, 09.04.2021 01:50



