20. There are two basic steps that take place when forming an ionic bond.
a) The formation of
...

Biology, 11.06.2020 20:57 didilove456
20. There are two basic steps that take place when forming an ionic bond.
a) The formation of
due to the transfer of electrons between
two atoms. In our example of sodium chloride (NaCl), sodium (Na) only has
electron in its valence shell and a lower electronegativity than
chlorine (CI), so Na will lose its valence electron to Cl. This produces a sodium
ion, with a
charge (Na+1). Chlorine has
electrons in its
valence shell and because of its higher electronegativity, gains an electron from
Na. This produces a chloride ion with a
charge (Cl-).
b) Once the ions have formed through the transfer of electrons, the
charges of the ions are attracted to one another, forming the
ionic bond.

Answers: 2
Another question on Biology

Biology, 22.06.2019 02:30
How can antibodies "remember" a particular bacterial invader or "antigen" on that invader?
Answers: 3

Biology, 22.06.2019 14:30
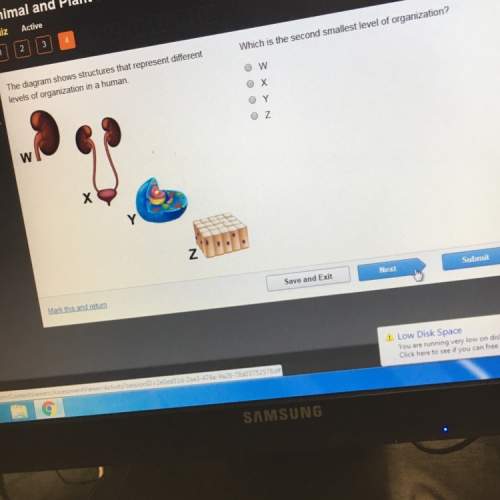
The diagram shows the development of the oocyte and the follicle during the menstrual cycle. identify at which stage in the cycle the hormone levels are at their highest and most active.
Answers: 1


Biology, 22.06.2019 22:30
Baroreceptors in the aortic arch and carotid bodies are mechanoreceptors that respond to the stretch that occurs in these arterial walls with changes in blood pressure. if pressure changes rapidly, the baroreceptors initiate a feedback mechanism that reduces blood pressure; however, if pressure changes slowly, as in developing hypertension, the receptors do not respond. given this information, the baroreceptors are most likely which type of receptor?
Answers: 3
You know the right answer?
Questions

Mathematics, 22.04.2021 20:10


Mathematics, 22.04.2021 20:10

Physics, 22.04.2021 20:10


Chemistry, 22.04.2021 20:10


Mathematics, 22.04.2021 20:10

Mathematics, 22.04.2021 20:10

Mathematics, 22.04.2021 20:10




Biology, 22.04.2021 20:10

Business, 22.04.2021 20:10


Chemistry, 22.04.2021 20:10


Mathematics, 22.04.2021 20:10

Mathematics, 22.04.2021 20:10