
Biology, 17.08.2020 22:01 cameron12502
A. Add to the formula in Interactive Question 3.5 to
show how increasing (CO2) dissolving in water leads
to a lower pH.
b. Use this formula to explain how a lower pH would
affect the [CO32-) in the ocean.
HCO3 SCO32- + H+
c. Assuming a fairly constant (Ca2+] in the ocean, how
would a change in (CO32-) affect the calcification
rate--the production of calcium carbonate
(CaCO3)-by the coral in a reef ecosystem?


Answers: 3
Another question on Biology

Biology, 21.06.2019 21:30
Organisms are classified, or grouped into categories, based on similarities in their characteristics and/or evolutionary relationships. the study of how organisms are classified is known as
Answers: 1

Biology, 22.06.2019 02:30
Contrast how pollination is different among gymnosperms and angiosperms.
Answers: 3

Biology, 22.06.2019 04:40
The negative impacts of nonnative species generally outweigh the positive impacts
Answers: 1

Biology, 22.06.2019 06:20
You see a white marker with an orange circle and black lettering. what does this marker tell you
Answers: 1
You know the right answer?
A. Add to the formula in Interactive Question 3.5 to
show how increasing (CO2) dissolving in water...
Questions




Mathematics, 16.02.2021 22:20

World Languages, 16.02.2021 22:20

Mathematics, 16.02.2021 22:20


Geography, 16.02.2021 22:20


Biology, 16.02.2021 22:20

Biology, 16.02.2021 22:20


Chemistry, 16.02.2021 22:20

Spanish, 16.02.2021 22:20


Spanish, 16.02.2021 22:20

Mathematics, 16.02.2021 22:20



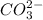 which easily reacts with water to form carbonic acid, thereby increasing water acidity.
which easily reacts with water to form carbonic acid, thereby increasing water acidity.


