Biology, 07.12.2019 13:31 fjjjjczar8890
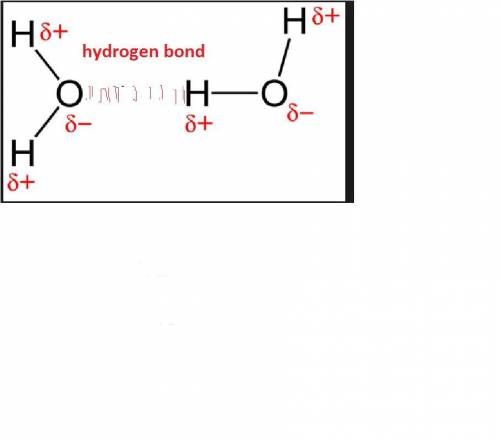
Which of the following best explains how hydrogen bonding affects the heat of vaporization for water?
a. the hydrogen bonds cause water to resist a change in temperature.
b. the number of hydrogen bonds in water increases the amount of h+ ions available.
c. the uneven pull of hydrogen bonds causes water molecules to repel each other.
d. the hydrogen bonds cause water to be highly attracted to other substances.

Answers: 3
Another question on Biology



Biology, 22.06.2019 02:00
Ateam from new york presbyterian hospital is conducting a study about a recent increase in cases involving both excess sweating and kidney stones. what would the team need before it draws a conclusion about the cases? medications that are specifically designed to treat the sickness machines that are built to process blood and reduce symptoms until patients are healthy again data that profile the various patients who report these symptoms proof that the excess sweating is related to the excessive kidney stones
Answers: 2

You know the right answer?
Which of the following best explains how hydrogen bonding affects the heat of vaporization for water...
Questions

Mathematics, 15.10.2019 14:50

Mathematics, 15.10.2019 14:50

Mathematics, 15.10.2019 14:50

Spanish, 15.10.2019 14:50


English, 15.10.2019 14:50


Mathematics, 15.10.2019 14:50

Mathematics, 15.10.2019 14:50



Mathematics, 15.10.2019 14:50


History, 15.10.2019 14:50

Mathematics, 15.10.2019 14:50

Physics, 15.10.2019 14:50


Physics, 15.10.2019 14:50