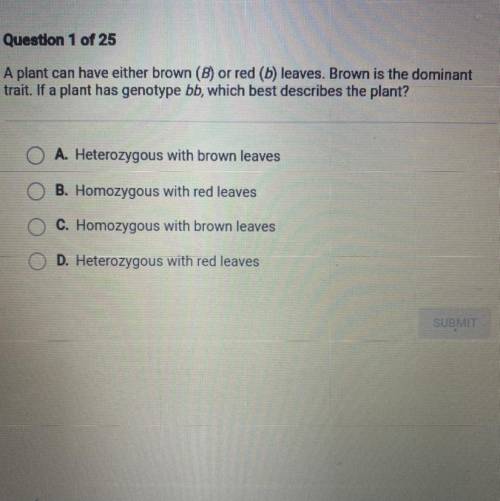
Can someone please look at the picture and help me?!
...

Answers: 1
Another question on Biology

Biology, 22.06.2019 05:20
Use this dichotomous key for insect identification to identify the insect shown. 1. a. insect has one pair of wings. order diptera b. insect has two pairs of wings. go to #2 2. a. front wings thicker in texture than hind wings go to #3. b. front and hind wings are same texture throughout. go to #4 3. a. front wings are short order dermaptera b. front wings cover entire abdomen order coleoptera 4. a. wings with scale on all parts of their area. order lepidoptera b. wings without scales go to #5. 5. a. hind wings smaller than front wings. order ephemeroptera b. front and hind wings nearly equal in size. order odonata the insect pictured is in the order diptera. ephemeroptera. coleoptera. odonata.
Answers: 3

Biology, 22.06.2019 06:00
What element is able to combine with itself and hydrogen to form large molecules ?
Answers: 1


Biology, 22.06.2019 08:40
What best explains whether bromine (br) or neon (ne) is more likely to form a covalent bond? bromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfills the octet rule. bromine forms covalent bonds because it has many electron shells, but neon has only two electron shells and is tightly bound to its electrons. neon forms covalent bonds because it can share its valence electrons, but bromine has seven valence electrons and can gain only one more electron. neon forms covalent bonds because it has only two electron shells, but bromine has many electron shells and will lose electrons in order to fulfill the octet rule.
Answers: 3
You know the right answer?
Questions

Social Studies, 10.07.2019 12:30


Mathematics, 10.07.2019 12:30


Mathematics, 10.07.2019 12:30


English, 10.07.2019 12:30





History, 10.07.2019 12:30

Social Studies, 10.07.2019 12:30

Social Studies, 10.07.2019 12:30



Social Studies, 10.07.2019 12:30