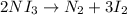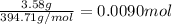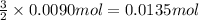Chemistry, 21.07.2019 06:00 itzmelanie1
The chemical equation below shows the decomposition of nitrogen triiodide (ni3) into nitrogen (n2) and iodine (i2). 2ni3 mc030-1.jpg n2 + 3i2 the molar mass of i2 is 253.80 g/mol, and the molar mass of ni3 is 394.71 g/mol. how many moles of i2 will form 3.58 g of ni3?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2

Chemistry, 23.06.2019 11:30
If this sedimentary rock layer is truly the oldest one of marine origin, what do you think that tells usabout the formation of earth's oceans?
Answers: 2
You know the right answer?
The chemical equation below shows the decomposition of nitrogen triiodide (ni3) into nitrogen (n2) a...
Questions

History, 27.05.2021 03:50


Mathematics, 27.05.2021 03:50

English, 27.05.2021 03:50


Mathematics, 27.05.2021 03:50



History, 27.05.2021 03:50

Mathematics, 27.05.2021 03:50

English, 27.05.2021 03:50

Mathematics, 27.05.2021 03:50


History, 27.05.2021 03:50



English, 27.05.2021 03:50


Mathematics, 27.05.2021 03:50

Chemistry, 27.05.2021 03:50