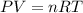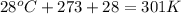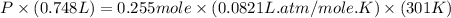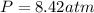Chemistry, 21.07.2019 04:00 robertstoll81
Question 1 a sample of 0.255 mole of gas has a volume of 748 ml at 28°c. calculate the pressure of this gas. (r= 0.0821 l ∙ atm / mol ∙ k) 0.784 atm 8.42 atm 0.00842 atm 7.84 × 10-4 atm none of the above

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
Question 1 a sample of 0.255 mole of gas has a volume of 748 ml at 28°c. calculate the pressure of t...
Questions

Business, 01.05.2021 16:30

English, 01.05.2021 16:30



Mathematics, 01.05.2021 16:30




Mathematics, 01.05.2021 16:30


English, 01.05.2021 16:30

English, 01.05.2021 16:30

Mathematics, 01.05.2021 16:30

Mathematics, 01.05.2021 16:30





Spanish, 01.05.2021 16:40