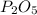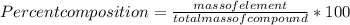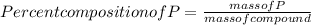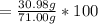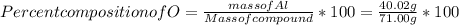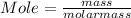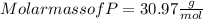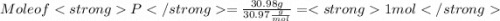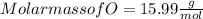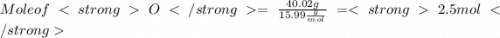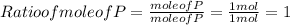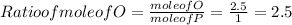Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1


Chemistry, 23.06.2019 06:30
What happens to the glucose molecule during the process of cellular respiration? (5 points) select one: a. it gets broken down. b. it forms oxygen. c. it builds muscles. d. it uses up energy.
Answers: 3

Chemistry, 23.06.2019 08:30
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1
You know the right answer?
When a 30.98-g sample of phosphorus reacts with oxygen, a 71.00-g sample of phosphorus oxide is form...
Questions








Mathematics, 28.11.2020 01:10

Mathematics, 28.11.2020 01:10


Biology, 28.11.2020 01:10

Mathematics, 28.11.2020 01:10








Advanced Placement (AP), 28.11.2020 01:10