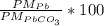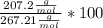Chemistry, 21.07.2019 03:00 ZachLaVine2016
Calculate the percentage by mass of lead in pbco3. a.17.96 b.22.46 c.73.05 d.77.54 e.89.22

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
Calculate the percentage by mass of lead in pbco3. a.17.96 b.22.46 c.73.05 d.77.54 e.89.22...
Questions


Biology, 31.01.2020 20:48

Social Studies, 31.01.2020 20:48

Spanish, 31.01.2020 20:48

Spanish, 31.01.2020 20:48



English, 31.01.2020 20:48

Health, 31.01.2020 20:48

Mathematics, 31.01.2020 20:49





History, 31.01.2020 20:49

Social Studies, 31.01.2020 20:49

Mathematics, 31.01.2020 20:49

Health, 31.01.2020 20:49


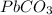 and Pb.
and Pb.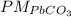 = 267,21
= 267,21 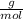
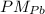 = 207,2
= 207,2