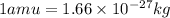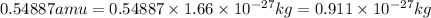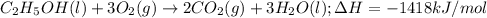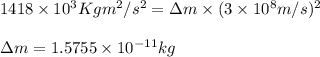3. when two atoms of 2h (deuterium) are fused to form one atom of 4he (helium), the total energy evolved is 3.83 × 10-12 joules. what is the total change in mass (in kilograms) for this reaction? 4. the mass of a proton is 1.00728 atomic mass units (amu) and the mass of a neutron is 60co nucleus whose nuclear mass is 1.00867 amu. what is the mass defect (in amu) of a 27 59.9338 amu? what is the mass defect in kilograms? what is the energy equivalent of this mass in kilojoules? 5. the equation shows one mole of ethanol fuel being burned in oxygen. convert the energy released into its equivalent mass. c2h5oh(l) + 3 o2(g) 2 co2(g) + 3 h2o (l) δh = -1418 kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Which of these reactions are redox reactions? check all that apply.cd + hcl → cdcl2 + h2cucl2 + na2s → 2nacl + cuscaco3 → cao + co2 2zns + 3o2 → 2zno + 2so2 ch4 + 2o2 → co2 + 2h2o
Answers: 3

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
3. when two atoms of 2h (deuterium) are fused to form one atom of 4he (helium), the total energy evo...
Questions


Biology, 17.05.2021 23:00


Mathematics, 17.05.2021 23:00







Advanced Placement (AP), 17.05.2021 23:00


Mathematics, 17.05.2021 23:00



Mathematics, 17.05.2021 23:00

Mathematics, 17.05.2021 23:00

Mathematics, 17.05.2021 23:00


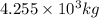
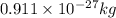 and energy equivalent to this mass is
and energy equivalent to this mass is 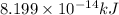
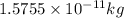

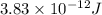
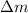 = mass change = ?
= mass change = ?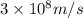


![\Delta m=[(n_p\times m_p)+(n_n\times m_n)+]-M](/tpl/images/0113/2964/f34f4.png)
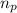 = number of protons = 27
= number of protons = 27
 = mass of one proton = 1.00728 amu
= mass of one proton = 1.00728 amu
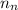 = number of neutrons = 33
= number of neutrons = 33
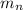 = mass of one neutron = 1.00867 amu
= mass of one neutron = 1.00867 amu
![\Delta m=[(27\times 1.00728)+(33\times 1.00867)]-[59.9338]\\\\\Delta m=0.54887amu](/tpl/images/0113/2964/c0b92.png)