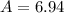Lithium-6 has a mass of 6.0151 amu and lithium-7 has a mass of 7.0160 amu. the relative abundance of li-6 is 7.42% and the relative abundance of li-7 is 92.58%. based on this data alone, calculate the average atomic mass for lithium to the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1
You know the right answer?
Lithium-6 has a mass of 6.0151 amu and lithium-7 has a mass of 7.0160 amu. the relative abundance of...
Questions



Chemistry, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

History, 12.12.2020 15:50

Chemistry, 12.12.2020 15:50

Law, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

Physics, 12.12.2020 15:50

English, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

History, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50


Mathematics, 12.12.2020 15:50



Social Studies, 12.12.2020 15:50


English, 12.12.2020 15:50

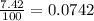
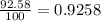
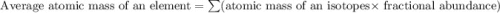
![A=\sum[(6.0151\times 0.0742)+(7.0160\times 0.9258)]](/tpl/images/0113/1342/3dcbf.png)