
Chemistry, 20.07.2019 21:30 leeahnnfoster
Butane c4 h10 (-1.jpghf = –125.7), combusts in the presence of oxygen to form co2 (g) (mc016-2.jpghf = –393.5 kj/mol), and h2 o(g) (mc016-3.jpghf = –241.82) in the reaction: mc016-4.jpg what is the enthalpy of combustion, per mole, of butane?

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1

Chemistry, 23.06.2019 17:10
What can form as a result of a chemical reaction? what can form as a result of a chemical reaction? compounds isotopes alpha particles beta particles
Answers: 2

Chemistry, 23.06.2019 17:40
When 7.00g of hydrogen react with 70.0g of nitrogen, hydrogen is considered the limiting reactant because
Answers: 1

Chemistry, 23.06.2019 18:20
In a chemical reaction, the number of moles of the reactants a. should never be equal to the number of moles of the products. b. may or may not be equal to the number of moles of the products. c. depends on the amount of product that is formed. d. should always be equal to the number of moles of the products.
Answers: 3
You know the right answer?
Butane c4 h10 (-1.jpghf = –125.7), combusts in the presence of oxygen to form co2 (g) (mc016-2.jpghf...
Questions

Social Studies, 10.02.2021 01:50


Mathematics, 10.02.2021 01:50


Mathematics, 10.02.2021 01:50




Social Studies, 10.02.2021 01:50

Geography, 10.02.2021 01:50

Mathematics, 10.02.2021 01:50

Chemistry, 10.02.2021 01:50

History, 10.02.2021 01:50

Mathematics, 10.02.2021 01:50

Mathematics, 10.02.2021 01:50

History, 10.02.2021 01:50


English, 10.02.2021 01:50


Biology, 10.02.2021 01:50

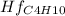 = -125.7 kJ/mol
= -125.7 kJ/mol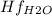 = -241.82 kJ/mol
= -241.82 kJ/mol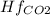 = -393.5 kJ/mol
= -393.5 kJ/mol

