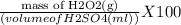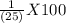Chemistry, 20.07.2019 20:00 phillipselijah2
A1.00 g sample of a hydrogen peroxide (h2o2) solution is placed in an erlenmeyer flask and diluted with 20 ml of 1 m aqueous sulfuric acid. to this solution is added 0.0200 m kmno4 solution via a buret, until a pale purple color persists. this requires 22.50 ml of kmno4 solution. what is the percent by mass of hydrogen peroxide in the original solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
You know the right answer?
A1.00 g sample of a hydrogen peroxide (h2o2) solution is placed in an erlenmeyer flask and diluted w...
Questions



History, 19.10.2020 05:01

Mathematics, 19.10.2020 05:01

English, 19.10.2020 05:01

English, 19.10.2020 05:01


Mathematics, 19.10.2020 05:01

Mathematics, 19.10.2020 05:01



Social Studies, 19.10.2020 05:01




Mathematics, 19.10.2020 05:01

Mathematics, 19.10.2020 05:01

Social Studies, 19.10.2020 05:01

History, 19.10.2020 05:01