Chemistry, 20.07.2019 19:00 martinez6221
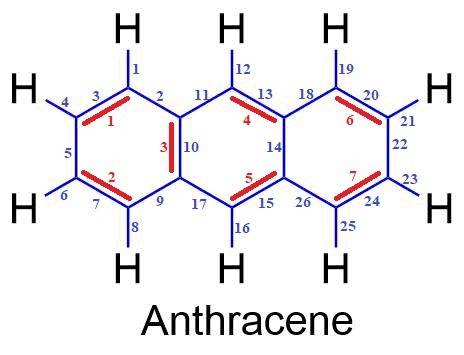
Anthracene is a yellow, crystalline solid found in coal tar. complete this structure for anthracene, c14h10, by adding bonds and hydrogen atoms as necessary. what type of hybrid orbitals are utilized by carbon in anthracene? how many σ bonds and π bonds are there in an anthracene molecule? how many valence electrons occupy σ-bond orbitals and how many occupy π -bond orbitals?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 23.06.2019 06:00
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2
You know the right answer?
Anthracene is a yellow, crystalline solid found in coal tar. complete this structure for anthracene,...
Questions



Physics, 11.10.2019 20:30




Mathematics, 11.10.2019 20:30


History, 11.10.2019 20:30

Mathematics, 11.10.2019 20:30

Chemistry, 11.10.2019 20:30



Spanish, 11.10.2019 20:30


French, 11.10.2019 20:30

Mathematics, 11.10.2019 20:30


Mathematics, 11.10.2019 20:30