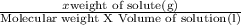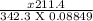Chemistry, 20.07.2019 16:00 carterjpowell77
Sucrose is very soluble in water. at 25◦c, 211.4 grams of sucrose will dissolve in 100 g of water. given that the density of the saturated sucrose solution is 1.34 g/ml, what is the molarity of the solution

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
Sucrose is very soluble in water. at 25◦c, 211.4 grams of sucrose will dissolve in 100 g of water. g...
Questions





Arts, 10.05.2021 18:10





Business, 10.05.2021 18:10


Mathematics, 10.05.2021 18:10

Mathematics, 10.05.2021 18:10

Mathematics, 10.05.2021 18:10

Social Studies, 10.05.2021 18:10

Chemistry, 10.05.2021 18:10

Mathematics, 10.05.2021 18:10

Mathematics, 10.05.2021 18:10