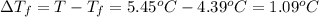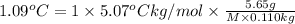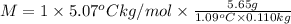Chemistry, 20.07.2019 15:30 emmanuelmashao5504
Asolution is made by dissolving 5.65 g of an unknown molecular compound in 110.0 g of benzene froze at 4.39 oc. what is the molar mass of the solute if pure benzene has a freezing point of 5.45 oc and the kf value of benzene is 5.07 oc/m

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2
You know the right answer?
Asolution is made by dissolving 5.65 g of an unknown molecular compound in 110.0 g of benzene froze...
Questions



Mathematics, 14.07.2021 15:50

Biology, 14.07.2021 15:50

English, 14.07.2021 15:50

Social Studies, 14.07.2021 15:50

Mathematics, 14.07.2021 15:50

Mathematics, 14.07.2021 15:50


Mathematics, 14.07.2021 15:50

Mathematics, 14.07.2021 15:50


Mathematics, 14.07.2021 15:50


Mathematics, 14.07.2021 15:50


Mathematics, 14.07.2021 15:50


Spanish, 14.07.2021 15:50

Mathematics, 14.07.2021 15:50

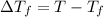
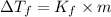
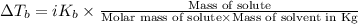
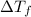 =Depression in freezing point
=Depression in freezing point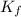 = freezing point constant of solvent= 5.07°C/m
= freezing point constant of solvent= 5.07°C/m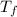 =4.39 °C
=4.39 °C