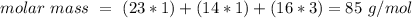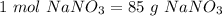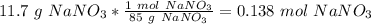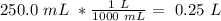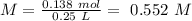Chemistry, 20.07.2019 15:00 saraaaaa0404
Determine the molarity of a solution made by dissolving 11.7 g of nano3 in water where the final volume of the solution is 250.0 ml.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Determine the molarity of a solution made by dissolving 11.7 g of nano3 in water where the final vol...
Questions


Mathematics, 03.12.2020 08:30

History, 03.12.2020 08:30

Mathematics, 03.12.2020 08:30



Spanish, 03.12.2020 08:30



Mathematics, 03.12.2020 08:30

Mathematics, 03.12.2020 08:30

Social Studies, 03.12.2020 08:30

Computers and Technology, 03.12.2020 08:30

Mathematics, 03.12.2020 08:30


Mathematics, 03.12.2020 08:30


Business, 03.12.2020 08:40

Health, 03.12.2020 08:40

Biology, 03.12.2020 08:40

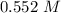
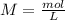
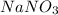 and the L of
and the L of