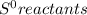Chemistry, 20.07.2019 13:30 FireStorm7327
What is the value for δs°reaction for the following reaction, given the standard entropy values c6h12o6(s) + 6o2(g) —-> 6co2(g) + 6h2o(l) +131 j/k -131 j/k +262j/k -262 j/k +417j/k

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
What is the value for δs°reaction for the following reaction, given the standard entropy values c6h1...
Questions


Physics, 26.09.2021 18:20

Chemistry, 26.09.2021 18:20


Social Studies, 26.09.2021 18:20

Mathematics, 26.09.2021 18:20



Biology, 26.09.2021 18:20


English, 26.09.2021 18:20

Arts, 26.09.2021 18:30

History, 26.09.2021 18:30

Mathematics, 26.09.2021 18:30

Mathematics, 26.09.2021 18:30

Mathematics, 26.09.2021 18:30


Mathematics, 26.09.2021 18:30


Mathematics, 26.09.2021 18:30

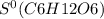 = 212.1 J/K.mol
= 212.1 J/K.mol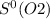 = 205.0 J/K.mol
= 205.0 J/K.mol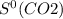 = 213.6 J/K.mol
= 213.6 J/K.mol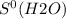 = 69.9 J/K.mol
= 69.9 J/K.mol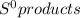 - ∑
- ∑