
Chemistry, 20.07.2019 00:30 xcncxgnfxg6487
The vapor pressure of pure ethanol at 60 °c is 0.459 atm. raoult's law predicts that asolution prepared by dissolving 10.0 mmol naphthalene (nonvolatile) in 90.0 mmol ethanol willhave a vapor pressure of atm.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2


Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
The vapor pressure of pure ethanol at 60 °c is 0.459 atm. raoult's law predicts that asolution prepa...
Questions



History, 30.11.2019 08:31

Social Studies, 30.11.2019 08:31

Computers and Technology, 30.11.2019 08:31



Chemistry, 30.11.2019 08:31





Chemistry, 30.11.2019 08:31



English, 30.11.2019 08:31




Advanced Placement (AP), 30.11.2019 08:31

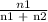 =
= 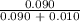 = 0.9
= 0.9

