Chemistry, 20.07.2019 00:00 jeffcarpenter
Atritium nucleus is formed by combining two neutrons and a proton. the mass of this nucleus is 9.106 × 10–3 universal mass unit less than the combined mass of the particles from which it is formed. approximately how much energy is released when this nucleus is formed.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
You know the right answer?
Atritium nucleus is formed by combining two neutrons and a proton. the mass of this nucleus is 9.106...
Questions

Mathematics, 30.04.2021 01:00




Mathematics, 30.04.2021 01:00


Mathematics, 30.04.2021 01:00

Health, 30.04.2021 01:00



Mathematics, 30.04.2021 01:00

English, 30.04.2021 01:00

Arts, 30.04.2021 01:00



Mathematics, 30.04.2021 01:00


Arts, 30.04.2021 01:00


English, 30.04.2021 01:00

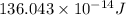
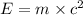
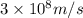
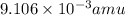
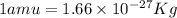
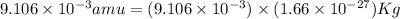
![E=[(9.106\times 10^{-3})\times (1.66\times 10^{-27})Kg]\times (3\times 10^8m/s)^2](/tpl/images/0109/7110/13e90.png)