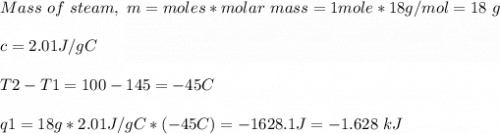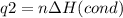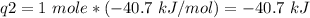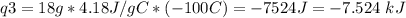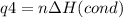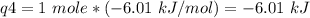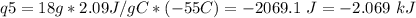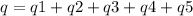Chemistry, 19.07.2019 21:00 wendelkristen
How much heat is evolved in converting 1.00 mol of steam at 145.0 ∘c to ice at -55.0 ∘c? the heat capacity of steam is 2.01 j/(g⋅∘c) and of ice is 2.09 j/(g⋅∘c).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

You know the right answer?
How much heat is evolved in converting 1.00 mol of steam at 145.0 ∘c to ice at -55.0 ∘c? the heat c...
Questions

Mathematics, 11.03.2021 22:20




Physics, 11.03.2021 22:20

Chemistry, 11.03.2021 22:20






Mathematics, 11.03.2021 22:20



Chemistry, 11.03.2021 22:20


Business, 11.03.2021 22:20

Mathematics, 11.03.2021 22:20


Mathematics, 11.03.2021 22:20