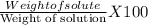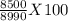Chemistry, 15.10.2019 13:30 GreenHerbz206
An aqueous solution has a mass of 490 grams containing 8.5×5103 gram of calcium ions. the concentration of calcium ions is in this solution is

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
An aqueous solution has a mass of 490 grams containing 8.5×5103 gram of calcium ions. the concentrat...
Questions





Mathematics, 25.09.2021 08:40

Mathematics, 25.09.2021 08:40

History, 25.09.2021 08:40

Mathematics, 25.09.2021 08:40

Chemistry, 25.09.2021 08:40

Chemistry, 25.09.2021 08:40

Mathematics, 25.09.2021 08:40

History, 25.09.2021 08:40


Mathematics, 25.09.2021 08:40

Social Studies, 25.09.2021 08:40

History, 25.09.2021 08:40

English, 25.09.2021 08:40

Physics, 25.09.2021 08:40