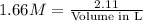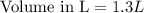Chemistry, 19.07.2019 17:00 dbn4everloved
How many liters of solution are needed to make a 1.66m solution containing 2.11 moles of kmno4

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

You know the right answer?
How many liters of solution are needed to make a 1.66m solution containing 2.11 moles of kmno4...
Questions







English, 14.12.2020 21:30


Mathematics, 14.12.2020 21:30

Mathematics, 14.12.2020 21:30

Mathematics, 14.12.2020 21:30

Mathematics, 14.12.2020 21:30

Mathematics, 14.12.2020 21:30


Mathematics, 14.12.2020 21:30

Geography, 14.12.2020 21:30




World Languages, 14.12.2020 21:30

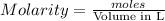
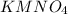 = 2.11 moles
= 2.11 moles