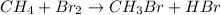Chemistry, 09.12.2019 19:31 wolfgirl10000
Given the equation: ch4 + br2 → ch3br + hbr which type of reaction does this equation represent

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
You know the right answer?
Given the equation: ch4 + br2 → ch3br + hbr which type of reaction does this equation represent...
Questions


Social Studies, 04.06.2021 23:10

Chemistry, 04.06.2021 23:10



Mathematics, 04.06.2021 23:10

Mathematics, 04.06.2021 23:10

Mathematics, 04.06.2021 23:10

Computers and Technology, 04.06.2021 23:10

Biology, 04.06.2021 23:10

Mathematics, 04.06.2021 23:10

Mathematics, 04.06.2021 23:10


Computers and Technology, 04.06.2021 23:10


Mathematics, 04.06.2021 23:10




English, 04.06.2021 23:10