
Chemistry, 19.07.2019 08:30 sabrinarasull1pe6s61
Assume you have 60 micrograms (1 microgram = 10-6 gram) of pure u-238. how many nuclei n0 is this? (recall avogadro's number equals 6.022×1023 particles per mole.)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2
You know the right answer?
Assume you have 60 micrograms (1 microgram = 10-6 gram) of pure u-238. how many nuclei n0 is this?...
Questions

Mathematics, 11.05.2021 19:50

Mathematics, 11.05.2021 19:50




Advanced Placement (AP), 11.05.2021 19:50


Mathematics, 11.05.2021 19:50




Biology, 11.05.2021 19:50

History, 11.05.2021 19:50


Business, 11.05.2021 19:50

Mathematics, 11.05.2021 19:50

Mathematics, 11.05.2021 19:50



Chemistry, 11.05.2021 19:50

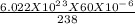 = 1.518 X 10^17 nuclei of Uranium.
= 1.518 X 10^17 nuclei of Uranium.

