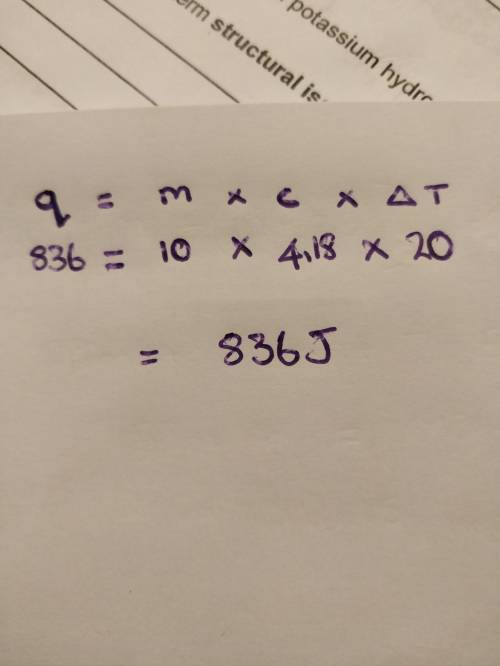Chemistry, 19.07.2019 06:00 johnkings140
What amount of heat is required to raise the temperature of 10.0 g of water from 15.0°c to 35.0°c? the specific heat of water is 4.18 j/g•°c. answer with 3 significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
What is the molar mass of babr2? a. 217.2 g/mol b. 297.1 g/mol c. 354.5 g/mol d. 434.4 g/mol
Answers: 1

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1


You know the right answer?
What amount of heat is required to raise the temperature of 10.0 g of water from 15.0°c to 35.0°c?...
Questions

Mathematics, 27.06.2019 21:00



Mathematics, 27.06.2019 21:00



Mathematics, 27.06.2019 21:00


Mathematics, 27.06.2019 21:00


History, 27.06.2019 21:00

Mathematics, 27.06.2019 21:00


Chemistry, 27.06.2019 21:00


Social Studies, 27.06.2019 21:00

Mathematics, 27.06.2019 21:00

Social Studies, 27.06.2019 21:00


Biology, 27.06.2019 21:00