Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which of the following elements is a representative element? a. chromium (cr) b. aluminum (al) c. mercury (hg) d. silver (ag)
Answers: 1


Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1
You know the right answer?
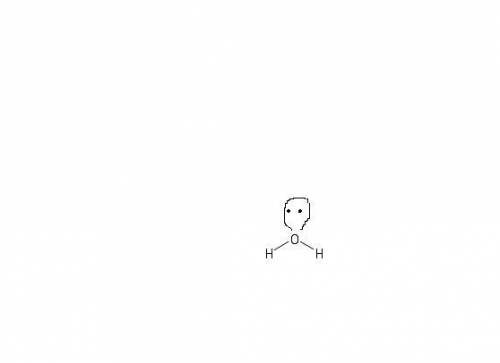
How does a lone pair contribute to molecular shape? a. it is too small to affect the molecule's sha...
Questions


Mathematics, 16.01.2020 15:31

Mathematics, 16.01.2020 15:31


Mathematics, 16.01.2020 15:31

Mathematics, 16.01.2020 15:31

Biology, 16.01.2020 15:31


Mathematics, 16.01.2020 15:31


English, 16.01.2020 15:31









English, 16.01.2020 15:31