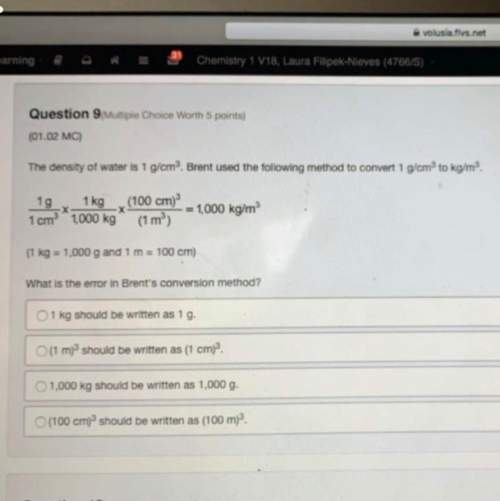
Compute the specific heat capacity at constant volume of nitrogen (n2) gas. the molar mass of n2 is 28.0 g/mol. c = j/(kg⋅k) request answer part b you warm 1.30 kg of water at a constant volume from 22.0 ∘c to 28.5 ∘c in a kettle. for the same amount of heat, how many kilograms of 22.0 ∘c air would you be able to warm to 28.5 ∘c? make the simplifying assumption that air is 100% n2. m = kg request answer part c what volume would this air occupy at 22.0 ∘c and a pressure of 1.10 atm ?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

You know the right answer?
Compute the specific heat capacity at constant volume of nitrogen (n2) gas. the molar mass of n2 is...
Questions



Advanced Placement (AP), 19.11.2020 19:00








History, 19.11.2020 19:00


English, 19.11.2020 19:00

Mathematics, 19.11.2020 19:00


English, 19.11.2020 19:00




Mathematics, 19.11.2020 19:00