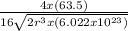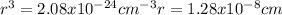Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 23.06.2019 07:00
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2
You know the right answer?
Copper has a face-centered cubic unit cell. the density of copper is 8.96 g/cm3. calculate a value f...
Questions

English, 16.10.2020 18:01

History, 16.10.2020 18:01


Health, 16.10.2020 18:01


Arts, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Geography, 16.10.2020 18:01

Physics, 16.10.2020 18:01

Computers and Technology, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01


Chemistry, 16.10.2020 18:01

French, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

English, 16.10.2020 18:01

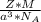
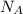 is Avogadro's number, and a is the edge length.
is Avogadro's number, and a is the edge length.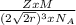
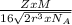
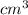 =
=