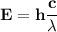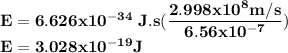Chemistry, 18.07.2019 09:00 hamada11617
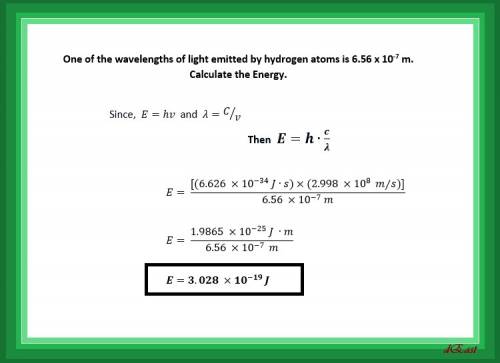
One of the wavelengths of light emitted by hydrogen atoms is 6.56 x 10-7 m. calculate the energy.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

You know the right answer?
One of the wavelengths of light emitted by hydrogen atoms is 6.56 x 10-7 m. calculate the energy....
Questions

Social Studies, 26.06.2019 08:30


Physics, 26.06.2019 08:30

Social Studies, 26.06.2019 08:30

Health, 26.06.2019 08:30


Social Studies, 26.06.2019 08:30



Mathematics, 26.06.2019 08:30


Mathematics, 26.06.2019 08:30



Social Studies, 26.06.2019 08:30



Biology, 26.06.2019 08:30

Physics, 26.06.2019 08:30

Biology, 26.06.2019 08:30