Chemistry, 19.11.2019 19:31 ladypink94
Asample of 1.6 g of methane (ch4) is completely burnt in 20.00 g of oxygen. the products are carbon dioxide and water. which is the excess reactant? which is the limiting reactant? how much of the excess reactant remains unreacted?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
Asample of 1.6 g of methane (ch4) is completely burnt in 20.00 g of oxygen. the products are carbon...
Questions

SAT, 30.12.2020 14:00


Mathematics, 30.12.2020 14:00

Social Studies, 30.12.2020 14:00


English, 30.12.2020 14:00

Business, 30.12.2020 14:00




English, 30.12.2020 14:00

Geography, 30.12.2020 14:00

Geography, 30.12.2020 14:00


Mathematics, 30.12.2020 14:00

Mathematics, 30.12.2020 14:00

English, 30.12.2020 14:00

Mathematics, 30.12.2020 14:00

Mathematics, 30.12.2020 14:00

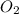
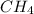
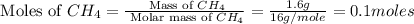
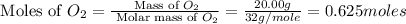
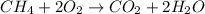
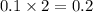 moles of
moles of