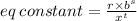Chemistry, 18.07.2019 03:00 baptistatm51976
In the reversible reaction shown below, r moles of a react with s moles of b to produce t moles of c. which equation can be used to represent the equilibrium constant for the forward reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
In the reversible reaction shown below, r moles of a react with s moles of b to produce t moles of c...
Questions

Social Studies, 24.05.2021 09:30




Mathematics, 24.05.2021 09:30


Mathematics, 24.05.2021 09:30




Mathematics, 24.05.2021 09:30


Mathematics, 24.05.2021 09:30

History, 24.05.2021 09:30


Mathematics, 24.05.2021 09:30

History, 24.05.2021 09:30

Mathematics, 24.05.2021 09:30