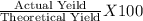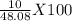Chemistry, 18.07.2019 00:30 njones58emailtjcedu
Hcl + naoh → nacl + h2o 1. if 30g of hcl is reacted with excess naoh, and 10g of nacl is produced, what is the theoretical yield of the experiment? 2. if 30 g of hcl is reacted with excess naoh, and 10 g of nacl is produced, what is the percent yield of the experiment?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 05:30
Find the midpoint of a segment with endpoints of 4-3i and -2+7i
Answers: 2

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1
You know the right answer?
Hcl + naoh → nacl + h2o 1. if 30g of hcl is reacted with excess naoh, and 10g of nacl is produced, w...
Questions


English, 22.01.2020 21:31

Business, 22.01.2020 21:31


Physics, 22.01.2020 21:31

Computers and Technology, 22.01.2020 21:31


Mathematics, 22.01.2020 21:31


Mathematics, 22.01.2020 21:31

Physics, 22.01.2020 21:31



English, 22.01.2020 21:31


History, 22.01.2020 21:31