
Chemistry, 17.07.2019 20:00 kyllow5644
Acompound has a percent composition of 40.0% carbon, 6.72% hydrogen and 53.28% oxygen. if its molar mass is 180 g/mol, what is its molecular formula?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
Acompound has a percent composition of 40.0% carbon, 6.72% hydrogen and 53.28% oxygen. if its molar...
Questions

Social Studies, 29.06.2019 00:50



Mathematics, 29.06.2019 00:50


Mathematics, 29.06.2019 00:50

English, 29.06.2019 00:50



Mathematics, 29.06.2019 00:50





Mathematics, 29.06.2019 00:50





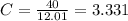
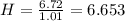
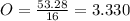
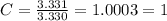
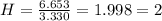
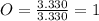
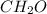
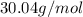
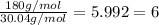
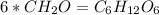
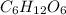 .
.


