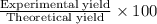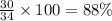Chemistry, 17.07.2019 13:00 ringo12384
When nh3 is prepared from 28 g n2 and excess h2, the theoretical yield of nh3 is 34 g. when this reaction is carried out in a given experiment, only 30. g is produced. what is the percentage yield?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
When nh3 is prepared from 28 g n2 and excess h2, the theoretical yield of nh3 is 34 g. when this rea...
Questions

Arts, 14.01.2021 18:50

Arts, 14.01.2021 18:50

Biology, 14.01.2021 18:50

Spanish, 14.01.2021 18:50

Chemistry, 14.01.2021 18:50

Health, 14.01.2021 18:50





Mathematics, 14.01.2021 18:50


Mathematics, 14.01.2021 18:50



Mathematics, 14.01.2021 18:50

History, 14.01.2021 18:50

Mathematics, 14.01.2021 18:50

Spanish, 14.01.2021 18:50

History, 14.01.2021 18:50