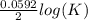Chemistry, 17.07.2019 08:00 crawford184232323234
What is the value of the equilibrium constant for this redox reaction? 2ag+(aq) + zn (s) → 2ag (s) + zn2+(aq) e = +1.56 v k=10(ne∘0.0592) k = 2.25 × 10-26 k = 2.25 × 1026 k = 5.04 × 10-52 k = 5.04 × 1052

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
What is the value of the equilibrium constant for this redox reaction? 2ag+(aq) + zn (s) → 2ag (s)...
Questions

English, 04.12.2019 15:31


Mathematics, 04.12.2019 15:31





Mathematics, 04.12.2019 15:31

Chemistry, 04.12.2019 15:31

Social Studies, 04.12.2019 15:31



Social Studies, 04.12.2019 15:31

English, 04.12.2019 15:31


Biology, 04.12.2019 15:31




 ,
,