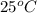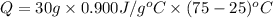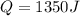Chemistry, 17.07.2019 05:00 rlumanlan549
The specific heat of aluminum is 0.900 j/g•°c. how much heat is required to raise the temperature of a 30.0 g block of aluminum from 25.0°c to 75.0°c?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
The specific heat of aluminum is 0.900 j/g•°c. how much heat is required to raise the temperature of...
Questions





History, 14.12.2021 08:30

Mathematics, 14.12.2021 08:30

Mathematics, 14.12.2021 08:30


Mathematics, 14.12.2021 08:30

History, 14.12.2021 08:30





Mathematics, 14.12.2021 08:30



History, 14.12.2021 08:30

Mathematics, 14.12.2021 08:30

Mathematics, 14.12.2021 08:30

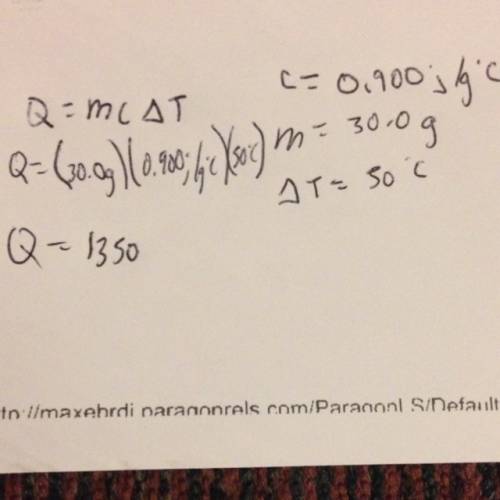
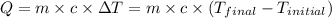
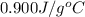
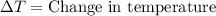
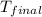 = final temperature =
= final temperature = 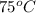
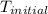 = initial temperature =
= initial temperature =