
Chemistry, 17.07.2019 01:30 tiffxnnyyy
What is the change in enthalpy for the following reaction? 2h2o2(aq) 2h2o(l) + o2(g) given: h2o: ∆h= -242 kj h2o2: ∆h= -609 kj

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3
You know the right answer?
What is the change in enthalpy for the following reaction? 2h2o2(aq) 2h2o(l) + o2(g) given: h2o:...
Questions



Mathematics, 07.11.2019 12:31

Mathematics, 07.11.2019 12:31

Mathematics, 07.11.2019 12:31

Mathematics, 07.11.2019 12:31





English, 07.11.2019 12:31


Mathematics, 07.11.2019 12:31


Mathematics, 07.11.2019 12:31

Mathematics, 07.11.2019 12:31



Mathematics, 07.11.2019 12:31

History, 07.11.2019 12:31

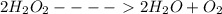 .
.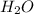 is -242 KJ
is -242 KJ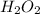 is -609 KJ.
is -609 KJ.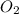 ] -
] - 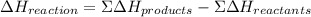
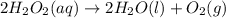
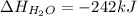
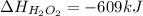
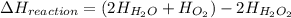
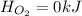 (as it is in its standard state)
(as it is in its standard state)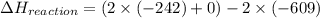
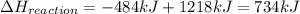
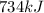 .
.

