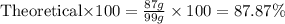Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
What is the percent yield of h2o if 87.0 g of h2o is produced by combining 95.0 g of o2 and 11.0 g o...
Questions


Physics, 23.08.2019 18:00

Mathematics, 23.08.2019 18:00


Mathematics, 23.08.2019 18:00

Mathematics, 23.08.2019 18:00

Mathematics, 23.08.2019 18:00

Social Studies, 23.08.2019 18:00

English, 23.08.2019 18:00


Mathematics, 23.08.2019 18:00



Social Studies, 23.08.2019 18:00

Biology, 23.08.2019 18:00

Physics, 23.08.2019 18:00


English, 23.08.2019 18:00

Biology, 23.08.2019 18:00

Social Studies, 23.08.2019 18:00

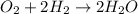
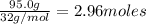
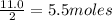
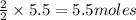 of water
of water