
Chemistry, 16.07.2019 09:30 manarsadi6
Achemical engineer determines the mass percent of iron in an ore sample by converting the fe to fe2+ in acid and then titrating the fe2+ with mno4−. a 1.3909−g sample was dissolved in acid and then titrated with 21.63 ml of 0.04462 m kmno4. the balanced equation is

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
Achemical engineer determines the mass percent of iron in an ore sample by converting the fe to fe2+...
Questions

Spanish, 03.12.2019 01:31

Biology, 03.12.2019 01:31

Mathematics, 03.12.2019 01:31

History, 03.12.2019 01:31







Health, 03.12.2019 01:31




Advanced Placement (AP), 03.12.2019 01:31



Biology, 03.12.2019 01:31

Social Studies, 03.12.2019 01:31

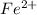 in acid and then titrating the
in acid and then titrating the 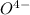 .
. 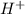 (aq) + 5
(aq) + 5 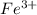 (aq) +
(aq) + 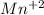 +(aq) + 4
+(aq) + 4 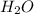 (l)
(l) 

