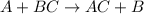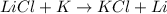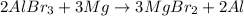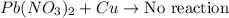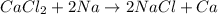Chemistry, 16.07.2019 08:30 Terrilady5
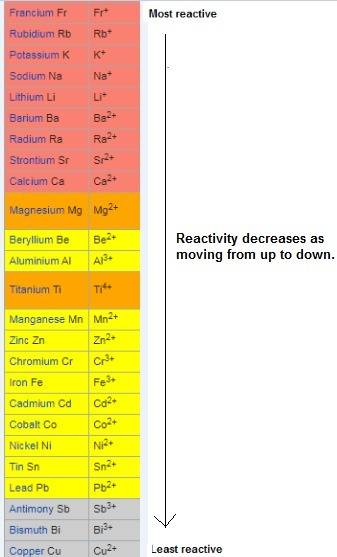
According to the activity series, which of the following single replacement reactions will take place? a. licl + k yields kcl + li b. 2 albr3 + 3mg yields 3mgbr2 + 2al c. pb(no3)2 + 2cu yields 2cuno3 + pb d. cacl2 + 2na yields 2nacl + ca

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:10
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
According to the activity series, which of the following single replacement reactions will take plac...
Questions


Computers and Technology, 04.07.2019 06:10

Computers and Technology, 04.07.2019 06:10




Chemistry, 04.07.2019 06:10

Advanced Placement (AP), 04.07.2019 06:10



Biology, 04.07.2019 06:10



Chemistry, 04.07.2019 06:20


Mathematics, 04.07.2019 06:20

Mathematics, 04.07.2019 06:20


Biology, 04.07.2019 06:20

Mathematics, 04.07.2019 06:20