
Chemistry, 16.07.2019 08:00 athenadailey8717
If 30.0 ml of 0.150 m aqueous sodium hydroxide is mixed with 30.0 ml of 0.150 m aqueous hydrochloric acid in a calorimeter at an initial temperature of 25.0 degrees celsius, what is the enthalpy change of this reaction if the final temperature reached in the calorimeter is 27.5 degrees celsius? naoh + hcl nacl + h2o

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1



Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
If 30.0 ml of 0.150 m aqueous sodium hydroxide is mixed with 30.0 ml of 0.150 m aqueous hydrochloric...
Questions

Social Studies, 23.06.2021 19:00



Mathematics, 23.06.2021 19:00



English, 23.06.2021 19:00





English, 23.06.2021 19:00

Mathematics, 23.06.2021 19:00



Biology, 23.06.2021 19:00

Physics, 23.06.2021 19:00

Chemistry, 23.06.2021 19:00

Mathematics, 23.06.2021 19:00

Mathematics, 23.06.2021 19:00


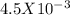 moles.
moles.

