
Chemistry, 16.07.2019 07:00 Arellano4672
For an exothermic dissolving process, predict the signs for the change in enthalpy, entropy, and free energy and explain how temperature will affect these changes.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
For an exothermic dissolving process, predict the signs for the change in enthalpy, entropy, and fre...
Questions



History, 05.11.2020 22:00


History, 05.11.2020 22:00


English, 05.11.2020 22:00


History, 05.11.2020 22:00


History, 05.11.2020 22:00

English, 05.11.2020 22:00

English, 05.11.2020 22:00

English, 05.11.2020 22:00


Mathematics, 05.11.2020 22:00


Health, 05.11.2020 22:00


History, 05.11.2020 22:00

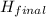 -
- 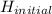 (
(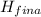 <
< 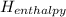 should always be negative.
should always be negative.

