
Chemistry, 16.07.2019 04:00 preciosakassidy
Asolution of an unknown nonvolatile nonelectrolyte was prepared by dissolving 0.250 g of the substance in 40.0 g of ccl4. the boiling point of the resultant solution was 0.357oc higher than that of the pure solvent. calculate the molar mass of the solute. (kb = 5.02oc/m)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
Asolution of an unknown nonvolatile nonelectrolyte was prepared by dissolving 0.250 g of the substan...
Questions


Biology, 23.06.2019 09:00

Mathematics, 23.06.2019 09:00

Mathematics, 23.06.2019 09:00


Mathematics, 23.06.2019 09:00

Mathematics, 23.06.2019 09:00

Mathematics, 23.06.2019 09:00

Physics, 23.06.2019 09:00


History, 23.06.2019 09:00


Mathematics, 23.06.2019 09:00

Computers and Technology, 23.06.2019 09:00


Mathematics, 23.06.2019 09:00


Health, 23.06.2019 09:00


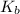 m
m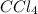 = 40,
= 40,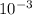 .
. 

