
Chemistry, 15.07.2019 21:30 paralaw61772
Calculate the mass of hcl required to prepare 2.5 liters of a 0.08 molar solution of hcl. 1 h 1.01 hydrogen 17 cl 35.45 chlorine a. 3.2 grams b. 4.5 grams c. 7.3 grams d. 11.4 grams

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

You know the right answer?
Calculate the mass of hcl required to prepare 2.5 liters of a 0.08 molar solution of hcl. 1 h 1.01 h...
Questions

Mathematics, 16.12.2020 20:30


Mathematics, 16.12.2020 20:30

Mathematics, 16.12.2020 20:30



Mathematics, 16.12.2020 20:30


History, 16.12.2020 20:30


Mathematics, 16.12.2020 20:30


Health, 16.12.2020 20:30

Mathematics, 16.12.2020 20:30

Mathematics, 16.12.2020 20:30

Chemistry, 16.12.2020 20:30

Mathematics, 16.12.2020 20:30

Mathematics, 16.12.2020 20:30


Chemistry, 16.12.2020 20:30

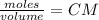
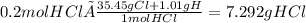 it's the choice c
it's the choice c

