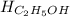Chemistry, 15.07.2019 19:30 CoolRahim9090
Calculate the standard molar enthalpy for the complete combustion of liquid ethanol (c2h5oh) using the standard enthalpies of formation of the reactants and products.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Calculate the standard molar enthalpy for the complete combustion of liquid ethanol (c2h5oh) using t...
Questions

Chemistry, 15.01.2021 07:10

Mathematics, 15.01.2021 07:10


Mathematics, 15.01.2021 07:10


English, 15.01.2021 07:10



Mathematics, 15.01.2021 07:10


Mathematics, 15.01.2021 07:10


Mathematics, 15.01.2021 07:10

Mathematics, 15.01.2021 07:10





Mathematics, 15.01.2021 07:10

Arts, 15.01.2021 07:10

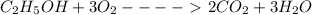
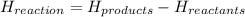
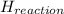 =
=